Overview
History
Vision and mandate
Where pCPA fits in the process
pCPA team
Overview
Through collective negotiations, the pan-Canadian Pharmaceutical Alliance has realized overall savings (as of April 2024) of $3.72 billion annually for brand name drugs and $914 million annually for generic drugs totaling $4.63 billion in annualized savings.
History
The pCPA was originally established as the pan-Canadian Pricing Alliance in August 2010. It was created by the premiers of Canada though the Council of the Federation’s Health Care Innovation Working Group. The aim was to achieve greater value for publicly funded drug programs and patients through the combined negotiating power of participating jurisdictions.
In 2015, the alliance was formalized with the new name pan-Canadian Pharmaceutical Alliance, a mandate and objectives were developed, a governance structure was implemented, and an office was created to provide support to the member jurisdictions. Also, in 2015, Québec joined the alliance and in 2016, the federal drug plans joined. At that time, Ontario agreed to host the pCPA Office and as such the Office was subject to Ontario rules and regulations. The pCPA Office was then staffed by employees of the Government of Ontario.
An organizational review conducted in 2019 to assess pCPA’s current and future roles recognized the importance of this rare collaboration of provincial, territorial and federal governments, which has enabled the sharing of resources and expertise to achieve its objectives. It also recommended that the pCPA become a standalone organization to better respond to the demands of the rapidly evolving pharmaceutical landscape. The transition to a standalone organization as well as other changes recommended by the organizational review were anchored in the pCPA’s 2022–24 strategic plan.
As a result of these collective efforts, the standalone pCPA organization was established in late 2022, and the transition was completed in 2023. The new organization builds on the important work accomplished by the dedicated team of pCPA staff. The new organizational structure, along with an increase in internal capacity, will allow the pCPA to lead and support more product negotiations on behalf of member jurisdictions in the years ahead.
pCPA member jurisdictions include public drug plan participation from British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland & Labrador, Yukon, Northwest Territories, Nunavut, Non-Insured Health Benefits (NIHB), Correctional Services of Canada (CSC), Veterans Affairs Canada (VAC) and Department of National Defence (DND).
Vision and mandate
Vision: To demonstrate collective leadership through value-driven investments in effective treatments that improve the health of Canadians and preserve a sustainable publicly funded health system in Canada.
Mandate: To conduct collective, expert-informed negotiations and achieve the pCPA objectives:
- Increase access to clinically relevant and cost-effective treatments
- Achieve consistent and lower drug costs
- Reduce duplication and optimize resource utilization
- Improve consistency in funding decisions
Where pCPA fits in the Canadian drug reimbursement process
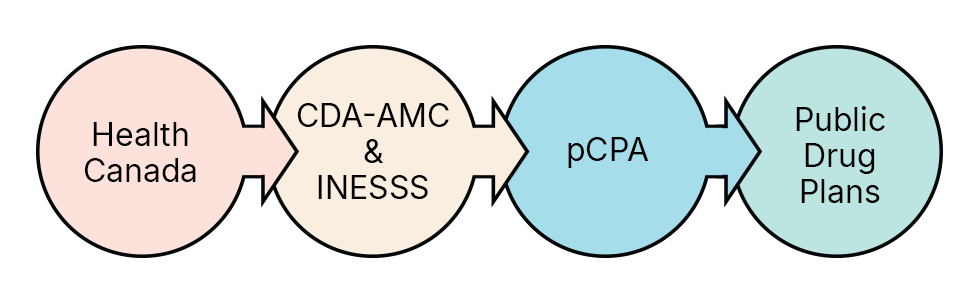
The pCPA is one part of the overall Canadian drug reimbursement process.
 *Please note that the figure above is a simplified illustration of the process and there can be exceptions.
*Please note that the figure above is a simplified illustration of the process and there can be exceptions.
Health Canada
Health Canada reviews drugs for safety, efficacy and quality before authorizing them for sale in Canada.
Canada's Drug Agency & INESSS
In Canada there are two health technology assessment organizations which review the clinical and cost-effectiveness of a drug product; Canada's Drug Agency (CDA-AMC, formerly CADTH) and in Québec, l’Institut national d’excellence en santé et en services sociaux (INESSS). The CDA-AMC and INESSS provide a recommendation to public drug plans on whether or not a drug should be reimbursed for public funding.
pCPA
The pCPA negotiation process begins for the majority of new drugs, once a recommendation is published by the CDA-AMC and/or INESSS. pCPA uses the recommendations from the CDA-AMC and INESSS and other factors to determine whether or not it will enter into a negotiation for a drug. Following a successful negotiation, pCPA will issue a letter of intent which sets the terms of the agreement between the pCPA and the drug manufacturer.
Public drug plans
Public drug plans make a final decision to fund a drug once a negotiation has been successfully completed and enter into a product listing agreement with the drug manufacturer.
pCPA team
The pCPA team works closely with the jurisdictions providing support in negotiations, administration, communications, standardization, analytics, process design, and policy related to brand and generic products.
Leadership team
CEO
Mauro Chies
Deputy CEO
Dominic Tan
Executive Director, Corporate Services
Gavrielle Tran
Executive Director, Communications
Genevieve C. Gagnon